Aquatest water hardness tester
for quick and easy determination of total water hardness
17,94 € exc. VAT
Water hardness is a property that expresses the content of dissolved minerals (most commonly CaO and MgO) in water. Water hardness is significant for its use as drinking and utility water. It is a source of lime scale formation and also influences the taste properties of water.
It can be permanent and temporary. Permanent contains dissolved chlorides, sulfides, nitrates, and silicates. Temporary contains dissolved Ca(HCO3)2 (calcium bicarbonate). Upon precipitation, CaCO3 (calcium carbonate) forms, which is lime scale.
Temporary water hardness can be removed by boiling, unlike permanent hardness.
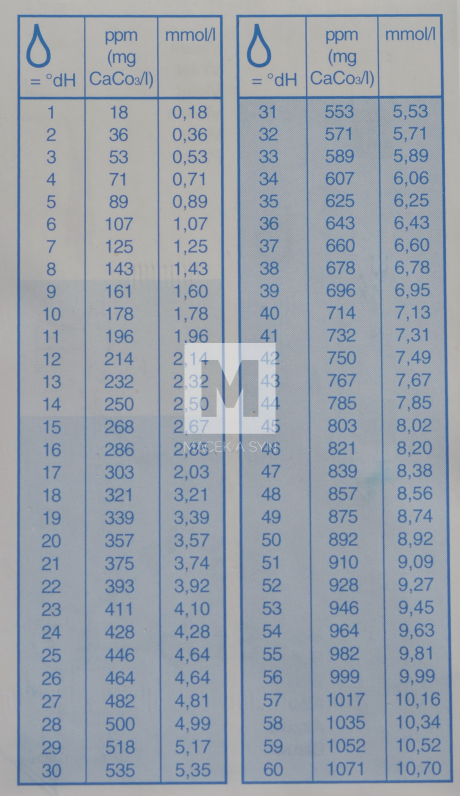
In the Czech lands, hardness was measured in German degrees, where one degree corresponds to 10 mg CaO per liter or 7.2 mg MgO per liter. According to current standards, it is expressed as the sum of calcium and magnesium in mmol/l. This standard was introduced relatively recently. 1 mmol/l corresponds to 5.61 German degrees. Water with hardness up to 0.7 mmol/l is considered very soft, above 3.75 mmol/l very hard.
CONVERSION OF WATER HARDNESS
| 1 mmol/l = 5,6 °dH | 1 °dH = 0,18 mmol/l |
| 1 mmol/l = 10 °F | 1 °F = 0,1 mmol/l |
| 1 °dH = 1,7 °F | 1 °F = 0,56 °dH |
- 1 °dH = German degree
- 1 °F = French degree
RANGES OF WATER HARDNESS
| Drinking water | mmol/l | °dH | °F |
| very hard | > 3,76 | > 21,01 | > 37,51 |
| hard | 2,51–3,75 | 14,01–21 | 25,01–37,5 |
| medium hard | 1,26–2,5 | 7,01–14 | 12,51–25 |
| soft | 0,7–1,25 | 3,9–7 | 7–12,5 |
| very soft | < 0,5 | < 2,8 | < 5 |